Data Science Services
State-of-the-art AI + data science
Save time with in-silico approaches
Extensive in-house tools, and experienced data science team
Embrace the future of drug product development with Leukocare. Our experienced team harnesses the power of AI and advanced data science for your drug development. We can speed up and de-risk your drug product development by leveraging cutting edge in-silico analysis.
Our advanced approach goes beyond conventional methods. We eliminate bias and de-risk your project using AI-recommended excipients from our Excipient Prediction Software (ExPreSo), a part of the comprehensive Leukocare Drug Development Software suite. You gain more insights per wet-lab experiment with our tailored Next-gen Design of Experiments (DoE) solutions from our expert team, significantly enhance drug substance understanding and deliver more comprehensive conclusions, even with limited drug substance availability.
At Leukocare we’re not just advancing drug development; we’re pioneering a smarter, more efficient path to success.
Leukocare’s Data Science expertise
Powerful bioinformatics at Leukocare
Take advantage of the power of bioinformatics with Leukocare's Drug Development Software, built upon extensive FDA and EMA published data and boosted by generative AI, which offers valuable support for formulation development. Utilizing our advanced data-science tools, you can
- Ensure unbiased, low-risk decision making by screening AI-recommended excipients, courtesy of our novel Excipient Prediction Software (ExPreSo).
- Deepen your understanding of your protein substance or facilitate candidate selection by quantitative analysis of surface properties relative to 800+ therapeutics, using the Finder of Liabilities and Structural Highlights (FLASH) tool.
- Identify excipients known to stabilize similar approved drug substances with our Similar Formulated Drug (SiFD) tool.
- Save time and effort answering critical questions on excipients and their concentrations in approved drugs. Leverage the tools Formulation Search and Excipient Concentrations, among others.
- Identify degradation pathways, and key molecular features to get a more comprehensive product understanding and choose the optimal drug candidate(s) through Molecular Modeling.
Leukocare’s superior biostatistical expertise empowers you to
- Significantly cut down on time, costs, and material by exploring the broadest design space using our Next-gen design of experiment (DoE), tailored precisely to your Target Product Profile (TPP) requirements.
- Optimize formulations by identifying the most stable excipient and buffer combinations in-silico by leveraging the predictive capabilities of Response Surface Methodology (RSM) modeling.
- Enhance long-term planning and increase your decision-making accuracy in predicting extended shelf life using linear regression, multiple regression, or advanced Kinetic Modeling.
Facilitate more informed decisions to save time and reduce risk
- Molecular characterization
- Identification of degradation pathways
- Stability assessment
- Data science-based excipient selection
- DoE to screen stabilizing effects and optimize excipients’ concentrations
- Response Surface Methodology (RSM) modeling to predict the most stabilizing combinations
- Linear regression
- Multiple regression
- Kinetic Modeling
Case study – Identification of degradation pathways
Identification of liability sites
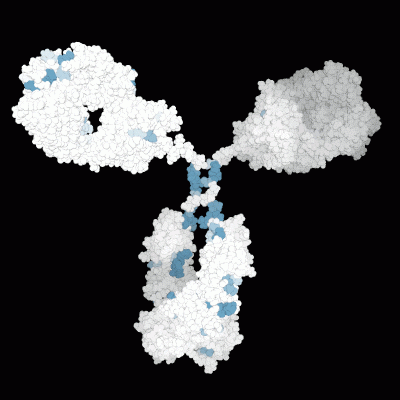
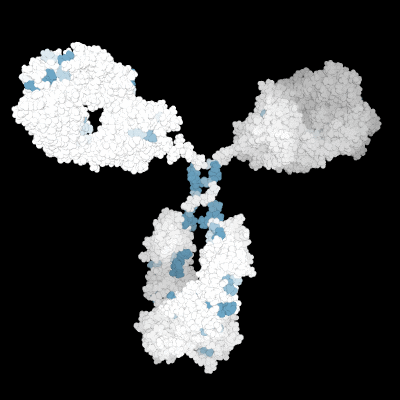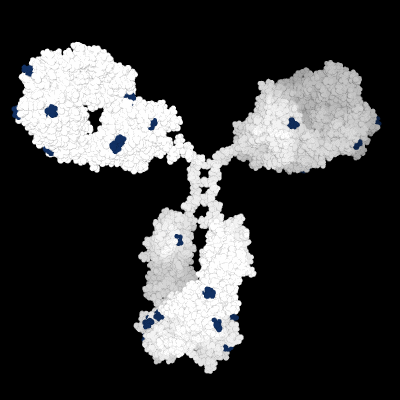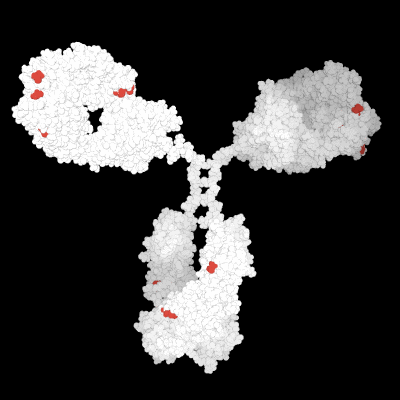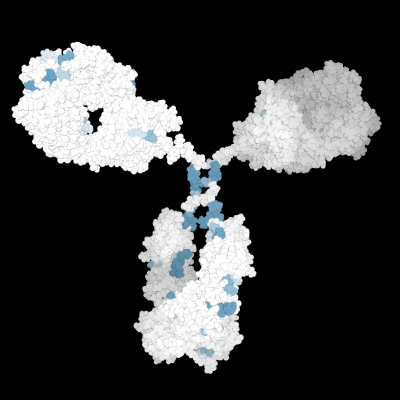
Prediction of the biological effects and behaviors of molecules.
Molecular Modeling of the 3D protein structure of an antibody highlighting various liability sites.
Case study – Data science for expedited excipient selection
Combination of DoE and accelerated aging
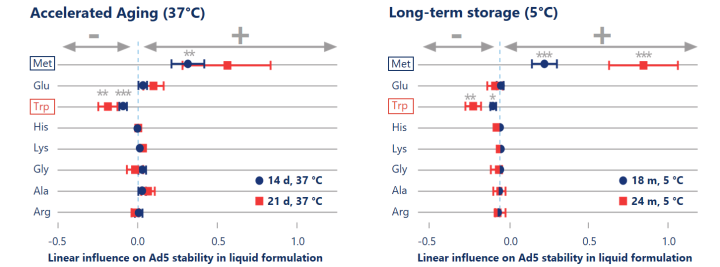
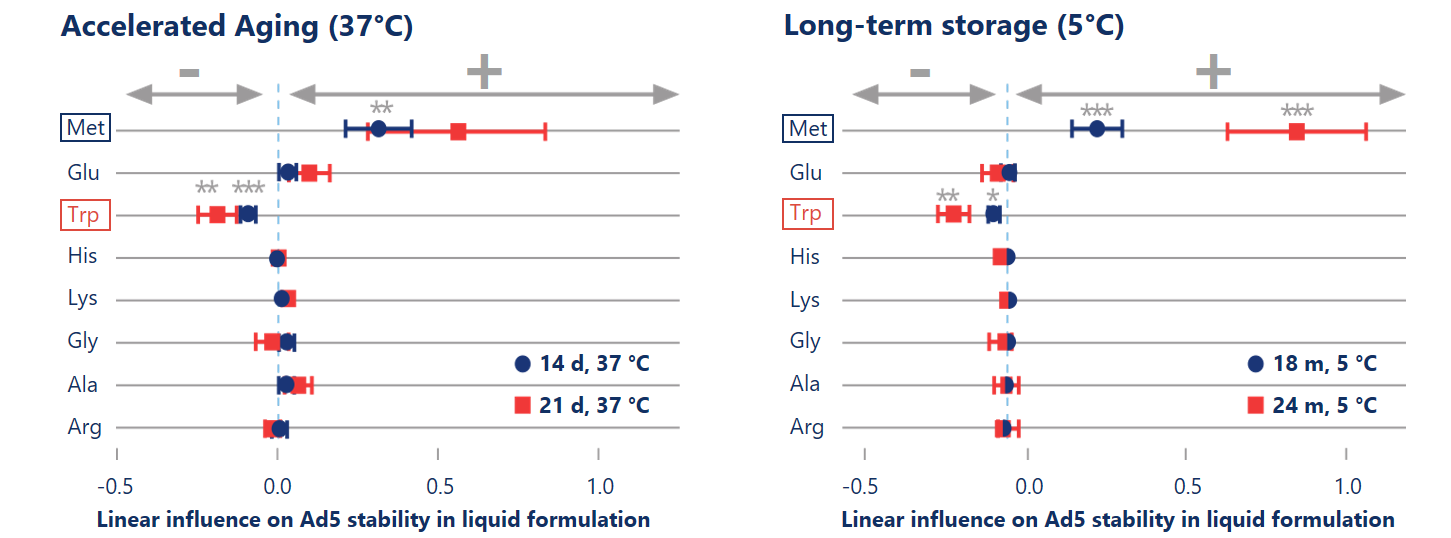
State-of-the-art data science speeds up the formulation development process of Ad5 in liquid formulation
DoE-based formulation development allowed us to explore the full design space of eight excipients with only 40 formulations and to apply regression analysis to statistically estimate the stabilizing effects of excipients. Accelerated aging at high temperature (37 °C, left) showed predictive power in detecting statistically significant (p< 0.01) stabilizing effects of methionine on the titer.
Reinauer et al. (2020), J Pharm Sci
On-demand Webinar

At Leukocare, we have developed a software called Excipient Prediction Software (ExPreSo), which is based on a machine-learning algorithm that suggests protective excipients based on the properties of the target drug substance and target product profile to improve excipient preselection. ExPreSo can suggest excipients for any drug substance with a peptide sequence.
In this webinar, Dr. Vidal will describe examples of protein surface properties derived from molecular modeling that proved important to ExPreSo, and how this confirms what is already known about molecular mechanisms underlying the excipient-mediated stabilization of biopharmaceuticals. Finally, she will discuss how ExPreSo can benefit Leukocare’s clients in the biopharmaceutical industry by reducing the time, cost, and risks associated with formulation development.
Watch the WebinarContact us
Speed up and de-risk your drug product development by leveraging cutting edge data science.
Contact usFlyer - Molecular Modeling
De-risk biologics development with molecular modeling.
Webinar - Developability Assessment
Learn how data science can support the nomination of a lead candidate molecule.